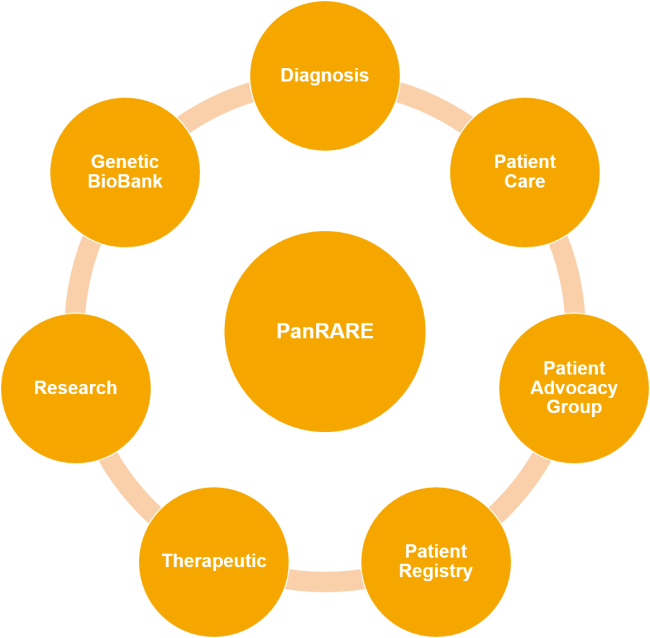
Overview of PanRARE CoE
PanRARE CoE is located in Hong Kong, Asia’s central city. The Center’s mission is to help both paediatric and adult patients who suffer from rare diseases. For example, in the neuromuscular disorders area, the PanRARE CoE provides expert opinion and care for patients who already have been diagnosed and/or are at risk of developing muscular dystrophy. We provide expeditious diagnostic, therapeutic evaluation, treatment options and references on a broad range of neuromuscular diseases including Duchenne/Becker Muscular Dystrophy, Myotonic Dystrophy I/II, Spinal Muscular Atrophy, Spinal Bulbar Muscular Atrophy (Kennedy’s Disease), Facioscapulohumeral Dystrophy, Limb Girdle Muscular Dystrophy Variants, Emery-Dreyfuss Muscular Dystrophy and Multiple Congenital Manifestations Of Muscular Dystrophies.
PanRARE CoE provides a platform to connect patients, doctors, academia, key opinion leaders, drug discovery and development enterprises and Patient Advocacy Group that specialize in muscular dystrophies and other rare diseases in order to achieve fast and accurate diagnosis for patient-specific treatment, supportive services, research and education.

























